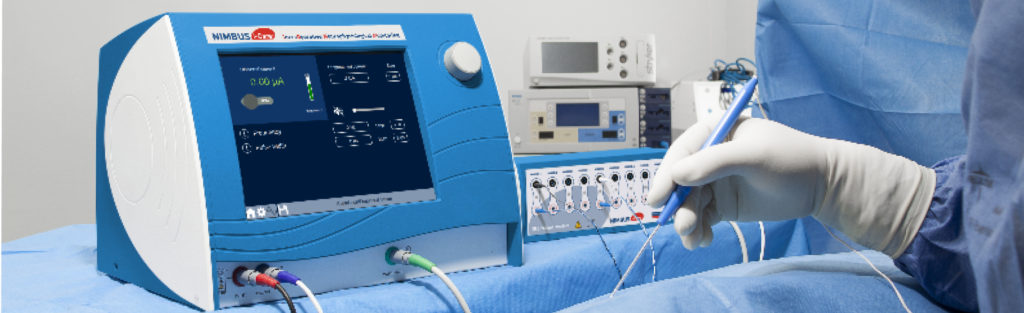
NIMBUS i-Care Lite, Neurostimulateur per-opératoire
Grâce au NIMBUS i-Care Lite, votre équipe chirurgicale pourra stimuler le système nerveux central et périphérique du patient.
Nos utilisateurs mondiaux ont approuvé et éprouvé la gamme i-Care depuis plus de 22 ans. De nombreuses publications scientifiques mentionnent son utilisation au bloc opératoire et démontrent sa sécurité au quotidien.
Neurostimulateur – Sécurisation des résultats chirurgicaux
L’utilisation du neurostimulateur per-opératoire au quotidien contribue à la sécurisation des résultats chirurgicaux et post-opératoire notamment en neurochirurgie4.
Les paramètres préconfigurés du neurostimulateur per-opératoire NIMBUS i-Care Lite permettent de sécuriser l’utilisation et d’améliorer l’opérationnalité de l’équipe chirurgicale. Ils ont été définis en s’appuyant sur la littérature scientifique et l’expérience d’utilisateurs afin d’aider à :
- Localiser les zones fonctionnelles du cortex et du sous-cortex par stimulation électrique directe ;
- Localiser et vérifier l’intégrité des fibres nerveuses (nerfs périphériques) par stimulation électrique directe.
Pour répondre à ces différents besoins, le neurostimulateur per-opératoire NIMBUS i-Care Lite offre deux principales configurations :
L’équipe chirurgicale suit auditivement la transmission de l’énergie de stimulation dans le patient en temps réel. Lors d’une stimulation des nerfs périphériques, les réactions musculaires spécifiques permettent de valider la localisation du nerf.
La neurostimulation per-opératoire avec NIMBUS i-Care Lite sécurise la qualité des résultats en préservant des faux négatifs et positifs grâce au suivi de l’impédance en continu.
NIMBUS i-Care Lite – Allie la spécificité et la simplicité de la neurochirurgie au quotidien
L’appareil NIMBUS i-Care Lite offre la possibilité de localiser les zones fonctionnelles du cortex et du sous-cortex par stimulation électrique directe. Il permet également de localiser, vérifier l’intégrité,
sectionner ou préserver les nerfs périphériques. Autant d’applications dédiées à la neurochirurgie depuis plus de 22 ans qui allie la robustesse (10 ans de durée de vie), la spécificité et la simplicité d’utilisation.
La création de paramètres personnalisables pour des équipes plus expertes améliore l’efficience de l’utilisation du NIMBUS i-Care Lite. Un accès rapide dans le menu principal permet de sélectionner le mode voulu et d’améliorer la rapidité d’installation. La simplicité de l’interface d’utilisation, le nombre limité de menus permettent un apprentissage rapide des équipes chirurgicales.
Une totale flexibilité est offerte grâce au choix du mode de stimulation, la caractérisation du type de signal émis et des paramètres d’utilisation. Une gamme variée d’électrodes de stimulation complète la flexibilité du NIMBUS i-Care Lite : nos électrodes monopolaires, bipolaires et tripolaires, droites ou coudées, offrent un large choix aux chirurgiens. Ainsi ce dernier peut adapter la sélectivité des électrodes de stimulation en fonction de ses besoins et de l’anatomie du patient.
L’accompagnement INNOPSYS et le réseau NIMBUS – L’optimisation des connaissances
Le personnel d’INNOPSYS forme les équipes médicales à une utilisation optimale du neurostimulateur per-opératoire NIMBUS i-Care Lite grâce au partage de ses connaissances. Ces formations en présentielles sont appuyées par des supports variés et des accompagnements pratiques. Des sessions de rafraîchissement à distance sont également planifiables.
Notre équipe vous accompagne également à distance pour toutes aides et demandes de diagnostic instantané liées à l’utilisation du NIMBUS i-Care Lite.
Le réseau d’utilisateurs de renom de la gamme i-Care offre la possibilité de partager des expériences, des connaissances et d’améliorer la coopération entre les services internationaux. Des visites en salle d’opération pour les chirurgies de pointe peuvent être organisées.

4. Rossi, M., Sciortino, T., Conti Nibali, M., Gay, L., Viganò, L., Puglisi, G., Leonetti, A., Howells, H., Fornia, L., Cerri, G., Riva, M., & Bello, L. (2021). Clinical Pearls and Methods for Intraoperative Motor Mapping. Neurosurgery, 88(3), 457–467. https://doi.org/10.1093/neuros/nyaa359
Applications
Spécifications Nimbus i-Care Lite
CLASSIFICATION ET NORMES APPLICABLES
| CLASSIFICATION ET NORMES APPLICABLES | |
|---|---|
| CERTIFICATION | NF EN ISO 13485:2016 |
| CLASSE DE RISQUE (MDD93/42/CEE, ANNEXE IX) | Classe IIa |
| ORGANISME NOTIFICATEUR | CE 0459 |
CARACTÉRISTIQUES TECHNIQUES
| SPÉCIFICATION ÉLECTRIQUE | ||
|---|---|---|
| RÉFÉRENCE | NICLSH | NICLSL |
| TENSION | 220 V - 230 V AC ±10% | 120 V AC ±10% |
| RACCORDEMENTS | ||
|---|---|---|
| ACCESSOIRES | Télécommande, rallonge de stimulation, valise de transport, câble secteur | |
| EXPORT DONNÉES | √ | |
| SYNCHRONISATION DU SIGNAL | √ | |
| PARAMÈTRES DE STIMULATION | ||
|---|---|---|
| FRÉQUENCE | 1-800 Hz | |
| LARGUEUR D'IMPULSION | 60-16000 µs | |
| INTENSITÉ DU COURANT | 0-32 mA | |
| MODE TRAIN « BURST » | √ | |
| DIMENSIONS ET POIDS (LxHxP) | ||
|---|---|---|
| NEUROSTIMULATEUR | 365 x 280 x 270 mm - 10,5 kg | |
Avant toute utilisation, merci de lire consciencieusement les instructions présentes dans le manuel d’utilisation. Les dispositifs médicaux sont des produits de santé, soumis à la règlementation Européenne et disposent du marquage CE 0459.